
Antibiotic resistance and the “end of modern medicine ”
Dr Siouxsie Wiles, Associate Professor, Molecular Medicine and Pathology and a Deputy Director of Te Pūnaha Matatini, a Centre of Research Excellence

Fungi photo credit: Shara Vab De OPas, Research Technician, Molecular Medicine and Pathology.
Background
Antibiotics are a cornerstone of modern medicine, used to treat people with infections as well as to prevent infections in those who are vulnerable, like people who need surgery or treatments like chemotherapy. Because bacteria are so good at adapting to their environment and picking up new genes from their surroundings, many are becoming resistant to the antibiotics that used to kill them. In 2014, the World Health Organization (WHO) reported that drug-resistant microbes are present in every region of the world. Their Director General at the time, Margaret Chan, called this issue “…the end of modern medicine as we know it”. The report concluded that within ten years, resistance would make routine surgery, organ transplantation, and cancer treatment life-threateningly risky. Around the world, people are already dying from antibiotic-resistant superbug infections.
Do novel antibiotics lie in fungi?
Dr Siouxsie Wiles and her team at the Bioluminescent Superbugs Lab are searching for new antibiotics in collaboration with Prof Brent Copp and Dr Melissa Cadelis from the University of Auckland’s School of Chemical Sciences and Dr Bevan Weir from the Crown Research Institute Manaaki Whenua. One of the first antibiotics ever discovered, penicillin comes from a family of fungi called Penicillium. Just like its flightless birds, Aotearoa New Zealand has fungi found nowhere else on Earth. Manaaki Whenua holds the International Collection of Microbes from Plants (ICMP) which has over 10,000 fungal cultures isolated from plants and soils from around New Zealand and the South Pacific and kept in frozen storage.
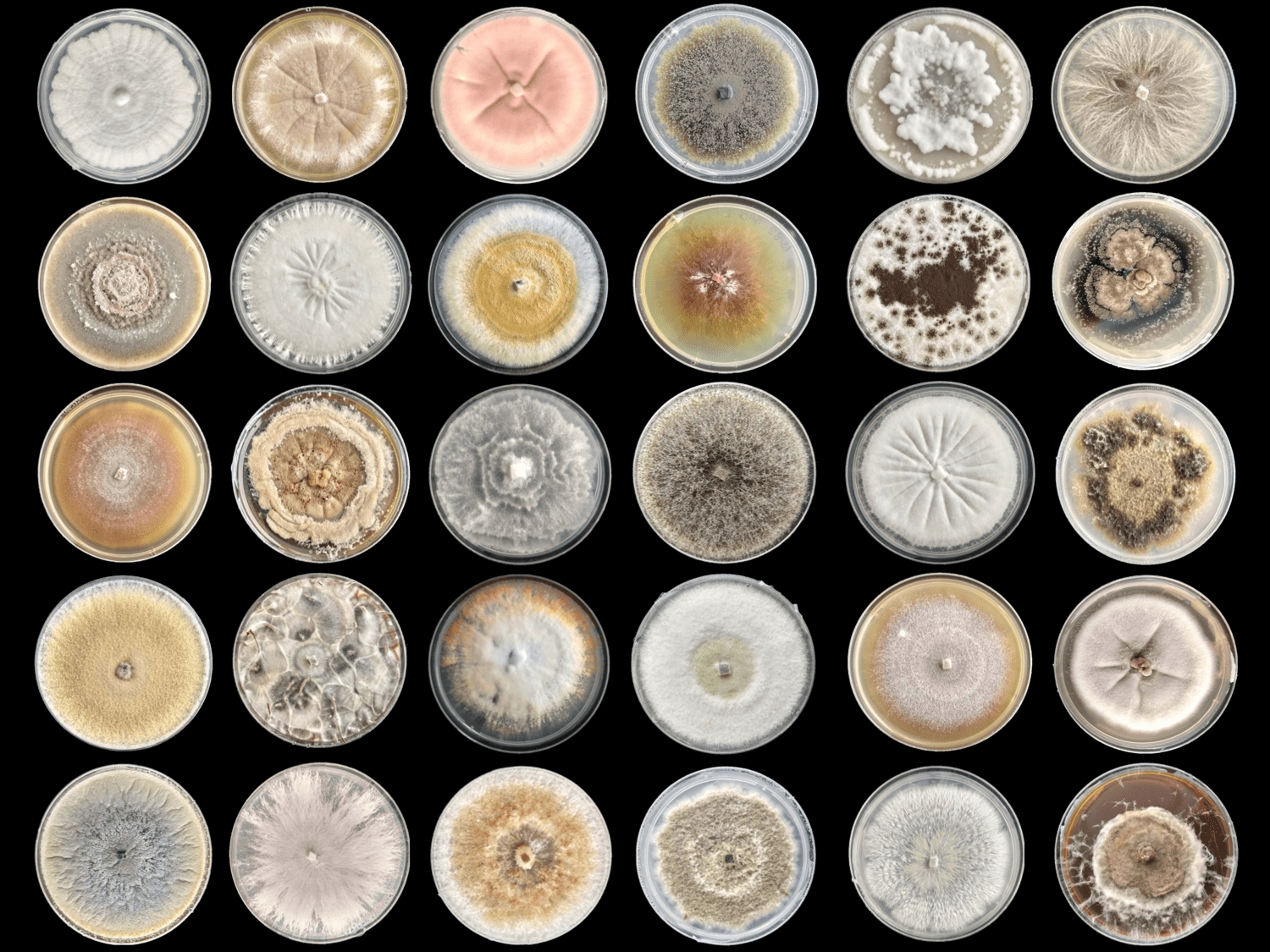
ICMP fungi growing in preparation for antibiotic-testing.
How did the Centre for eResearch help?
At the Bioluminescent Superbugs Lab, Siouxsie and her team use bacteria that have been engineered to glow in the dark to screen fungi from the ICMP collection for antibacterial activity. They test lots of different parameters, including growing the fungi on different media and for different amounts of time. Their experiments generates a large amount of data, so they were keen to work with the Centre for eResearch to come up with a better way of visualising it. The visualisation webpage and interactive graphs that the Centre for eResearch developed allow Siouxsie and her team to combine data from various lab members and to look at specific fungi, or media, or incubation times, as well as bacterial species we are testing against, all with a few clicks of the mouse. The screenshots below are examples of the interactive web that show a combination of data by selecting different criteria and from different researchers.
This project is currently funded by a generous donation from New Zealand Carbon Farming, and all the people who sponsor a fungus or who
Dr Wiles is passionate about demystifying science and making her research more publicly available. In 2017 she published her first book ‘Antibiotic resistance: the end of modern medicine?’ in which she describes the looming crisis of antibiotic resistance and its threat to New Zealand. During COVID-19 Dr Wiles joined forces with Spinoff cartoonist Toby Morris to make the science of the pandemic clear and understandable. Releasing their work under a Creative Commons licence, their graphics have been translated into multiple languages and have been adapted by various governments and organisations as part of their official pandemic communications.
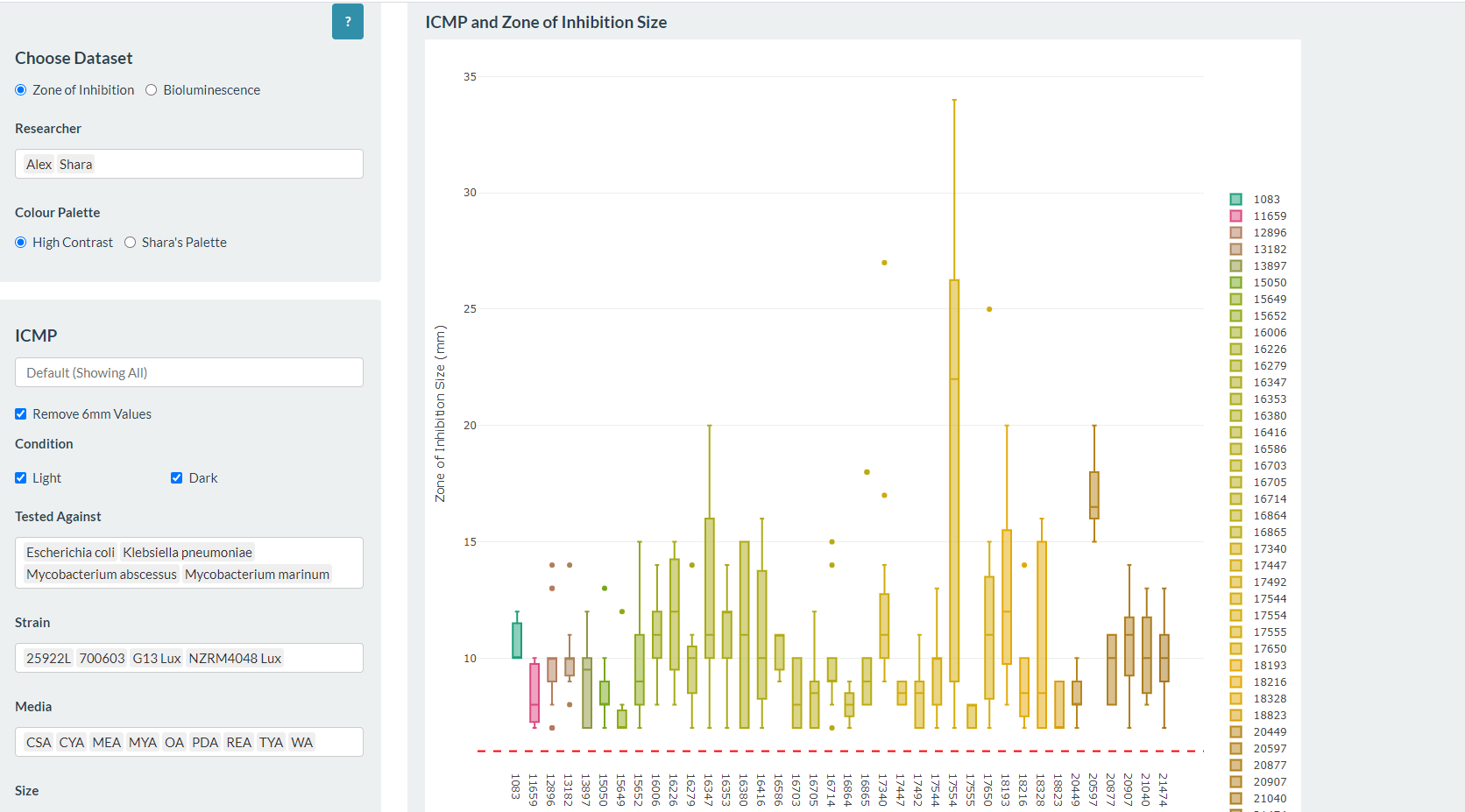
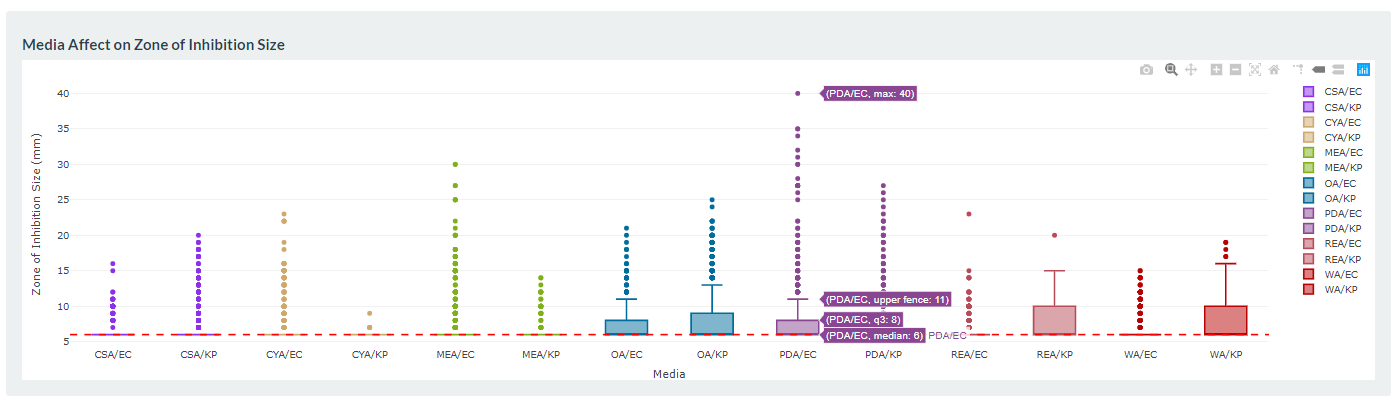
Interactive web page showing graphs from fungi/antibiotics research data.
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































