
Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke
William K. Diprose, MBChB 1,2 James, P. Diprose, BSc PhD 3 Michael T. M. Wang, MBChB 1 Gregory P. Tarr, MBChB, PhD 4 Andrew McFetridge, MBChB 4 P. Alan Barber, MBChB PhD FRACP 1,2 (see notes for authors affiliation)
Introduction
The treatment for Ischemic stroke caused by large vessel occlusion1,2 in patients has been revolutionised by endovascular thrombectomy techniques. Nevertheless, over one third of treated patients are deceased or become dependent on others for daily activities three months1 after the surgery. Finding improvement methods3 to identify patients who are most likely to be benefited from thrombectomy are needed, in order to avoid costly4, unnecessary and potentially dangerous procedure exposed to patients.
In this study, we hypothesized that an automated method of measuring intracranial CSF volume from baseline non-contrast computed tomography (CT) brains would predict outcome in patients undergoing endovascular thrombectomy for ischemic stroke. We predicted that patients with higher proportional volume of intracranial CSF (more cerebral atrophy) would tend to have worse outcomes than those with lower proportional volume of intracranial CSF (less cerebral atrophy)
This preliminary analysis is to look at whether a particular imaging feature from CT scans can predict outcome in patients who have strokes and undergo clot retrieval.
Methodology
Patients and definitions
The study center is a tertiary hospital that provides thrombectomy services to approximately 2.8 million people. We include consecutive patients from a single-center prospective endovascular thrombectomy registry. The attending stroke team assessed the baseline and 24-hour stroke severity with the National Institutes of Health Stroke Scale (NIHSS). A trained research nurse determined the mRS scores after contacting patients through phone at three-month time after surgery.
Image processing
We gathered files of non-contrast CT brains in the form of Digital Imaging and Communications in Medicine (DICOM) from the regional hospital Picture Archiving and Communication System. The selected de-identified axial CT brain DICOMs were loaded onto a virtual machine and pre-processed with Pydicom (Copyright 2008-2018, Darcy Mason and pydicom contributors). Intracranial tissue was segmented from the skull using the validated U-Net model and pre-trained weights published by Akkus et al.5 A U-Net is a type of deep convolutional neural network specifically designed for image segmentation tasks.6 The U-Net was run with Keras 2.2.4,7 and TensorFlow 1.9.0,8 on a graphics processing unit. The intracranial CSF was then segmented from the remaining intracranial tissue using Hounsfield unit (HU) thresholding, with a range of 0-15 HU inclusive, as described by Nguyen et al.9 Total 3-dimensional intracranial volume (including CSF) and 3-dimensional intracranial CSF volume were calculated by multiplying the number of pixels in the segmentation masks with the respective DICOM scan slice thickness and pixel spacing metadata. Intracranial CSF volume was reported as a proportion of total intracranial volume.10 An example of image segmentation is shown in Figure 1.
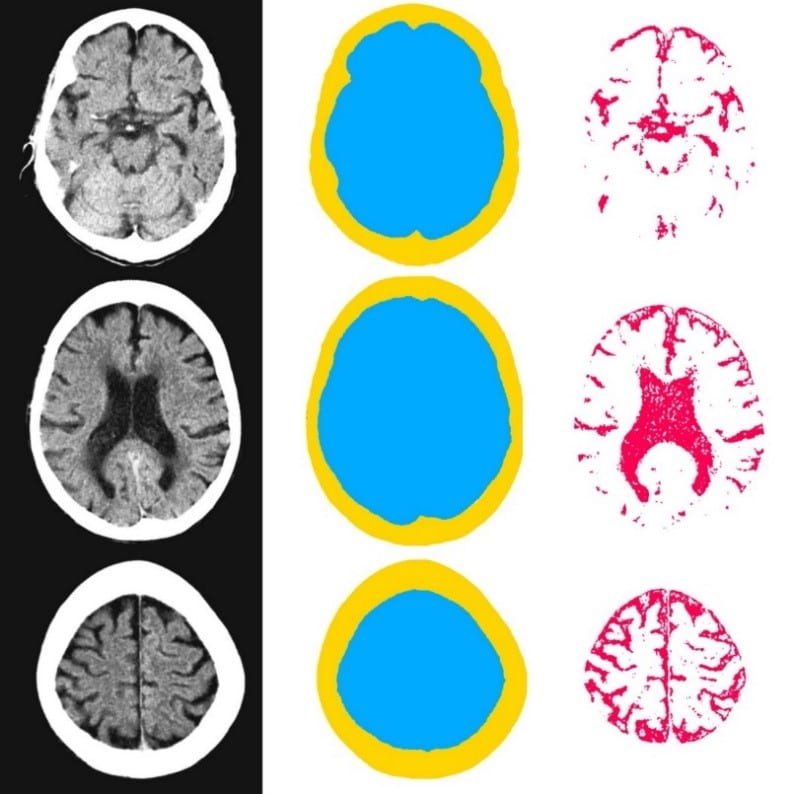
Figure 1. Image segmentation. Representative slices of non-contrast computed tomography brain (left). Blue (middle) indicates the intracranial mask which was segmented from the skull (yellow) using the validated U-Net published by Akkus et al.5 Red (right) indicates the intracranial cerebrospinal fluid which was segmented from the intracranial tissues using Hounsfield unit thresholding.
Defining outcomes
The primary outcome was the proportion of patients achieving functional independence at three months, defined by a mRS score of 0 to 2. Secondary outcomes included (1) the ordinal shift of mRS scores; (2) early neurological improvement, defined as a reduction in NIHSS of 8 or more points from baseline to 24 hours, or an NIHSS score of 0 or 1 at 24 hours; and (3) mortality at three months.
Statistical analysis
We used IBM SPSS Statistics version 22.0 (New York, USA) and GraphPad Prism version 6.02 (California, USA) to perform a statistical analysis. Preliminary univariate logistic or ordinal regression and Fisher’s exact tests were used to identify potential clinical predictors of primary and secondary outcome measures. The relationship between intracranial CSF volume and outcome measures was then assessed using multiple logistic or ordinal regression, incorporating relevant variables with p<0.15 from the univariate analysis. The number of variables used in the multivariable regression analysis was approximately limited to the number of adverse events divided by 10, to avoid overfitting. We then constructed a receiver operating characteristic (ROC) curve to assess the discriminative ability of intracranial CSF volume in predicting 3-month functional independence, and calculated Youden-optimal threshold prognostic accuracy values. All tests were two-tailed, and p<0.05 was considered statistically significant. The ethics required for the study has been approved by the regional ethics committee.
Results
In this analysis, there are 367 patients in total (221 men; mean±SD age 65.1±15.4 years, median [IQR] baseline NIHSS score of 17 [12-21]). Intravenous alteplase was administered to 195 (53.1%) patients. Successful recanalization (modified Thrombolysis in Cerebral Infarction grade 2b-3) was achieved in 321 (87.5%) patients, and 167 (45.5%) exhibited early neurological improvement. The median (IQR) 3-month mRS score was 2 (1- 4), with 205 (55.9%) patients achieving functional independence, and 61 (16.6%) patients deceased. The mean±SD intracranial CSF volume was 9.1±4.9%, and the range was 1.1 to 23.5%. Patients with higher intracranial CSF volumes were older, had poorer baseline function, and higher rates of hypertension, dyslipidemia, coronary artery disease, and atrial fibrillation. Unadjusted univariate and multivariable-adjusted odds ratios (ORs) of outcome measures by intracranial CSF volume. Multiple logistic and ordinal regression demonstrated that increasing intracranial CSF volume was a significant predictor of reduced functional independence (OR=0.68 per 5% increase; 95% confidence interval [CI], 0.50-0.91; P=0.01) and increased (worse) 3-month mRS scores (common OR=1.54; 95% CI, 1.04-2.28; P=0.03). The predictive ability of intracranial CSF volume measurements for functional independence was significantly greater than chance (AUC=0.577; 95% CI, 0.518-0.636; P=0.01), and the Youden-optimal prognostic cut-off was an intracranial CSF volume of <16%. The distribution of 3-month mRS scores by whether participants fulfilled this prognostic threshold is illustrated in Figure 2.

Figure 2. Distribution of modified Rankin Scale (mRS) scores at 3 months by intracranial cerebrospinal fluid (CSF) volume.
Discussion
For every 5% increase in intracranial CSF volume there was 32% lower odds of functional independence and 54% higher odds of a worse mRS score. When dichotomized, the intracranial CSF volume that discriminated outcome the best was <16%, with 60% and 34% of patients achieving functional independence in the <16% and >16% groups respectively. These findings suggest that cerebral atrophy may have an impact on outcome following endovascular thrombectomy and could improve patient selection in combination with other factors. This is consistent with a recent study in endovascular thrombectomy patients, where pre-existing small vessel disease burden, mainly driven by visually-graded cerebral atrophy, was associated with worse functional outcome.11
A logistic regression model developed using 500 patients from the MR CLEAN trial found that the baseline variables that predict a good functional outcome included:
- age,
- NIHSS score,
- pre-stroke mRS score,
- previous stroke,
- diabetes mellitus,
- systolic blood pressure,
- intravenous alteplase administration,
- Alberta Stroke Program Early CT Score,
- location of intracranial occlusion,
- CT angiogram collateral score and the estimated time from stroke onset to groin puncture.12
However, this model had only moderate discriminative ability with a C-statistic of 0.73, and it has not been taken up in clinical decision-making.3 In routine clinical practice, imaging features of infarct size, penumbral size, and cerebral blood flow are used to support decision-making,13 but no features of baseline ‘brain reserve’ are used.
We need to consider a number of methodological limitations when interpreting the findings of this study. Firstly, a U-Net may have resulted in better anatomical segmentation of the CSF. Secondly, a number of other pathologies may mimic high or low CSF volume, such as prior infarct, encephalomalacia, hydrocephalus or cerebral edema.14,15 Finally, the single-center retrospective design could have potential selection bias, hence limits the generalizability of the findings.
In summary, this study found that increased automated intracranial CSF volume was associated with worse outcome following endovascular thrombectomy. The mechanism leading to the worse outcome may be a consequence of poor brain reserve in patients with cerebral atrophy. Identifying patients who are unlikely to be benefited from endovascular thrombectomy remains an important challenge.3 Future studies should focus on validating intracranial CSF volume, or related markers of cerebral atrophy as predictors of outcome in endovascular thrombectomy patients. Finally, the addition of intracranial CSF volume to existing prediction tools for thrombectomy should be evaluated in future studies.
Acknowledgements: The authors gratefully thank the Neurological Foundation of New Zealand and the University of Auckland Centre for eResearch; in particular Jason He, Sina Masoud-Ansari and Sean Matheny for access and support to the virtual machine that enabled this research.
Reference
- Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110.
- Goyal M, Almekhlafi MA, Cognard C, McTaggart R, Blackham K, Biondi A, et Which patients with acute stroke due to proximal occlusion should not be treated with endovascular thrombectomy? Neuroradiology. 2019;61:3–8.
- Campbell BCV, Mitchell PJ, Churilov L, Keshtkaran M, Hong K-S, Kleinig TJ, et Endovascular Thrombectomy for Ischemic Stroke Increases Disability-Free Survival, Quality of Life, and Life Expectancy and Reduces Cost. Front. Neurol. 2017;8:657.
- Akkus Z, Kostandy PM, Philbrick KA, Erickson BJ. Extraction of brain tissue from CT head images using fully convolutional neural networks. In: Medical Imaging 2018: Image Processing. International Society for Optics and Photonics; 2018. p. 1057420.
- Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In: Medical Image Computing and Computer-Assisted Intervention– MICCAI 2015. Springer International Publishing; 2015. p. 234–241.
- Chollet, F. Keras. https://github.com/fchollet/keras,
- Abadi M, Barham P, Chen J, Chen Z, Davis A, Dean J, et al. Tensorflow: A system for large-scale machine learning. In12th Symposium on Operating Systems Design and Implementation; 2016. p. 265-283.
- Nguyen HS, Patel M, Li L, Kurpad S, Mueller W. Quantitative estimation of a ratio of intracranial cerebrospinal fluid volume to brain volume based on segmentation of CT images in patients with extra-axial hematoma. Neuroradiol. J. 2017;30:10–14.
- Wanifuchi H, Shimizu T, Maruyama T. Age-related changes in the proportion of intracranial cerebrospinal fluid space measured using volumetric computerized tomography scanning. J. Neurosurg. 2002;97:607–610.
- Arba F, Testa GD, Limbucci N, Nappini S, Renieri L, Pracucci G, et al. Small vessel disease and clinical outcomes after endovascular treatment in acute ischemic stroke. Neurol. Sci. 2019;40:1227–1235.
- Venema E, Mulder MJHL, Roozenbeek B, Broderick JP, Yeatts SD, Khatri P, et Selection of patients for intra-arterial treatment for acute ischaemic stroke: development and validation of a clinical decision tool in two randomised trials. BMJ. 2017;357:j1710.
- Román LS, Menon BK, Blasco J, Hernández-Pérez M, Dávalos A, Majoie CBLM, et Imaging features and safety and efficacy of endovascular stroke treatment: a meta- analysis of individual patient-level data. Lancet Neurol. 2018;17:895–904.
- Zhang A, Kao PY, Shelat A, Sahyouni R, Chen J, Manjunath BS. Fully Automatedm Volumetric Classification in CT Scans for Diagnosis and Analysis of Normal Pressure Hydrocephalus. arXiv preprint arXiv:1901.09088. 2019.
- Dhar R, Yuan K, Kulik T, Chen Y, Heitsch L, An H, et al. CSF Volumetric Analysis for Quantification of Cerebral Edema After Hemispheric Infarction. Neurocrit. Care. 2016;24:420–427.
Note: Author institutions:
- Department of Medicine, Faculty of Medical and Health Sciences, The University of Auckland, New Zealand
- Department of Neurology, Auckland City Hospital, Auckland, New Zealand
- Independent Computer Scientist, New Territories, Hong Kong
- Department of Radiology, Auckland City Hospital, Auckland, New Zealand
- Corresponding author: Professor P. Alan Barber
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































