
Calcium signalling in salivary gland acinar cells
Dr. John Rugis1, Professor James Sneyd1 Department of Mathematics, University of Auckland; David Yule2, Medical Center, University of Rochester.
The primary role of salivary gland acinar cells is to secrete saliva, the lack of which causes a host of severe medical difficulties. Thus, an understanding of the mechanisms underlying saliva secretion is vital for the understanding of oral health. In every kind of salivary acinar cell, saliva secretion is controlled by the concentration of cytosolic Ca2+. Our research group has constructed a spatiotemporal model of Ca2+ dynamics and luminal fluid flow in parotid acinar cells, based on new data about the distribution of inositol trisphophate receptors (IPR).
We have demonstrated that coupling fluid flow secretion with the Ca2+ signalling model changes the dynamics of the Ca2+ oscillations significantly, which indicates that Ca2+ dynamics and fluid flow cannot be accurately modelled independently. Further, we determined that an active propagation mechanism based on calcium-induced calcium release channels is needed to propagate the Ca2+ wave from the apical region to the basal region of the acinar cell. A graphical summary or our model is shown in figure 1.
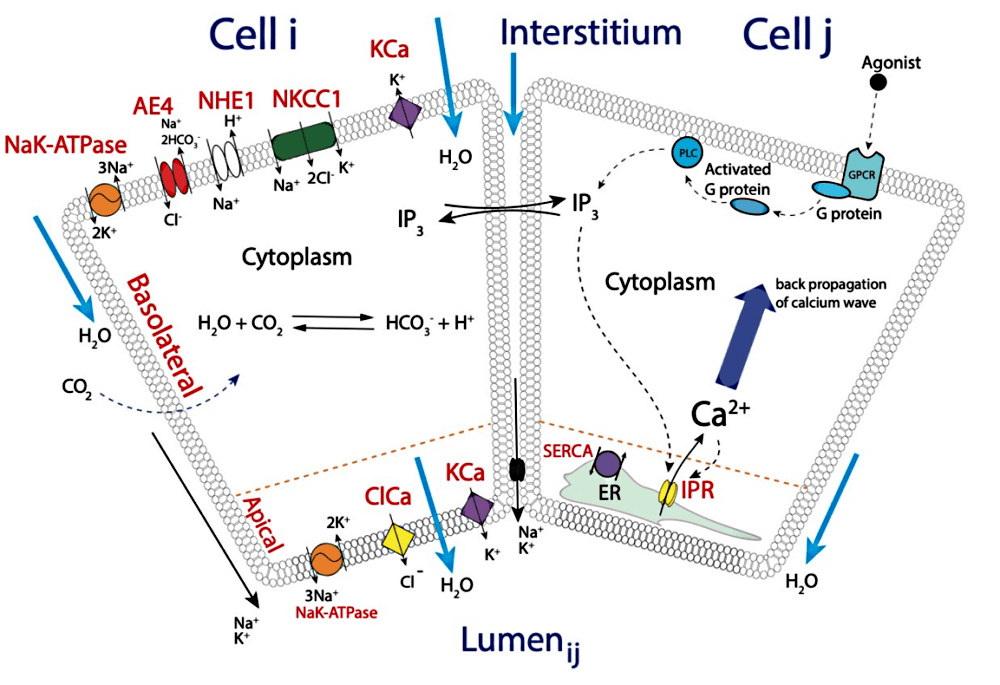
Figure 1 – Graphical representation of the calcium signalling and fluid flow model.
We constructed a three-dimensional anatomically accurate multicellular structural mesh model of a parotid gland acinus to investigate the effects of the topology of both its cells and lumen have on primary fluid secretion. The mesh consists of seven individual cells, coupled via conformal common luminal surfaces. Our mathematical model consists of a system of reaction-diffusion partial differential equations and our model is solved numerically on the mesh using the finite element method. A picture of our 3D seven-cell mesh is shown in figure 2.
We reused our mesh model and constructed an animated interactive augmented reality presentation of simulation results to facilitate interpretation and understanding. Figure 3 shows James Sneyd in the Centre for eResearch Visualisation Suite exploring simulation results.
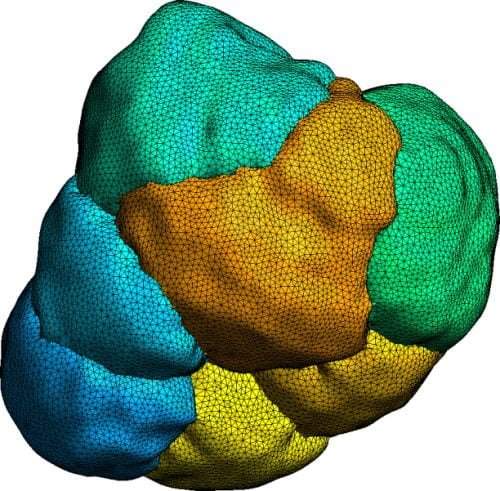
Figure 2 – Seven cell structural mesh, used for numerical modelling.

Figure 3 – Calcium signalling and fluid flow simulation results displayed in interactive 3D.
Reference
- Pages, N., Vera-Sigüenza E, Rugis, J., Kirk, V., Yule, D. I., & Sneyd, J. (2019). A Model of Ca2+ Dynamics in an Accurate Reconstruction of Parotid Acinar Cells. Bulletin of mathematical biology, 81 (5), 1394-1426
- Vera-Sigüenza E, Pages, N., Rugis, J., Yule, D. I., & Sneyd, J. (2019). A Mathematical Model of Fluid Transport in an Accurate Reconstruction of Parotid Acinar Cells. Bulletin of mathematical biology, 81 (3), 699-721
- Vera-Sigüenza E, Pages, N., Rugis, J., Yule, D. I., & Sneyd, J. (2020). A Multicellular Model of Primary Saliva Secretion in the Parotid Gland. Bulletin of mathematical biology, 82 (3)
Acknowledgement
Our work is heavily reliant on the computer resources and consultation services provided by both the Centre for eResearch and NeSI (New Zealand eScience Infrastructure). Quite simply put, we could not have extended our project to its current scope and depth without these resources. Special thanks to Nick Young of the Centre for eResearch for helping us create a Hololens visualisation movie showing our simulation results.
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































