
Computational investigation of catalysis mechanisms for polyurethane synthesis
Dr. Tilo Soehnel1, Senior Lecturer, Neil Edmonds2, Senior Lecturer, Ransi Devendra3, PhD candidate. 1 & 3 School of Chemical Sciences, 2 Department of Mechanical Engineering
Introduction
Polyurethanes are polymers made with a series of urethane linkages formed as a result of reaction between a polyisocyanate and a polyol1. Catalysis is an important part of polyurethane technology as it promotes the reaction between an isocyanate and an alcohol group to give the urethane linkage. Depending on the ratio of isocyanate to alcohol the final polymer will have different end groups. The products with isocyanate end groups are called pre-polymers and they are intermediates used in some polyurethane application. Catalysts used in pre-polymer preparation also play a role in the performance of the pre-polymer system in final applications. However the present knowledge on polyurethane catalysis cannot explain all behaviours of polyurethane systems. In this investigation mechanisms of various catalysts were investigated.
A complete understanding of the reaction mechanism allows for better control of the reaction at each stage. Experimental methods for investigating reaction mechanisms have been investigated by many researches to elucidate each step of the reaction. However, these investigations are mainly based on analyses of products formed in a chemical reaction using varying concentrations of reactants without considering the intermediates that are formed. This procedure can miss some of the important data related to the reaction and provide false leads on the actual mechanism. Computational techniques based on quantum mechanical methods can give information related to interactions between reactants and intermediates formed in a chemical reaction. These also have the ability to investigate specific interactions between selected functional groups to give a better insight as to intermediates formed. The most useful outcome is the ability to visualize the interactions, this gives an opportunity to test possible reaction pathways involved in reactions.
NeSI Pan cluster
In this project, the quantum chemistry software package Gaussian 09 available on the NeSI Pan cluster was used for computational modelling to investigate catalysis reaction pathways for urethane formation using electronic structure theory. In these investigations, DFT and ab-initio computational methods were used. Since it is possible to carry out several jobs at any given time, many different experiment models were simulated to relate catalysis of urethane formation. This feature gave the ability to achieve results in a fraction of the time compared to performing the investigation on a desktop computer.
Investigation into urethane catalysis
Organotin carboxylates are one of the common types of catalyst in synthesis of polyurethane used industrially. These catalysts are used in the synthesis of polyurethanes based on both aliphatic and aromatic isocyanates. It is reported in literature2 that organotin carboxylate interact with alcohol to give an organotin alkoxide which becomes the dominant catalyst for urethane formation. It is also believed that the same mechanism is true for both aliphatic and aromatic isocyanates. However, computational and experimental model compounds studied showed that organotin carboxylates do not undergo alkoxide formation by interacting with alcohol. A result of the computer simulation is shown in Figure1; this simulation does not show a transition state that would indicate the formation of an organotin alkoxide intermediate. Modelling, however, shown that the urethane formation takes place through a triple molecular interaction between organotin carboxylate, alcohol and the isocyanate3 as shown in Figure 2.

Figure 2: Three molecular interaction: (a) van der waals complex
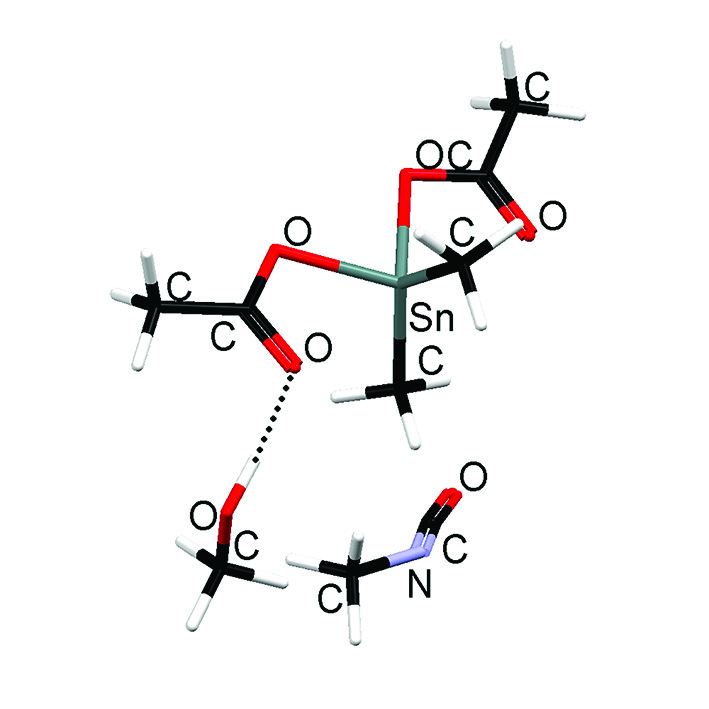
(b) transition state.
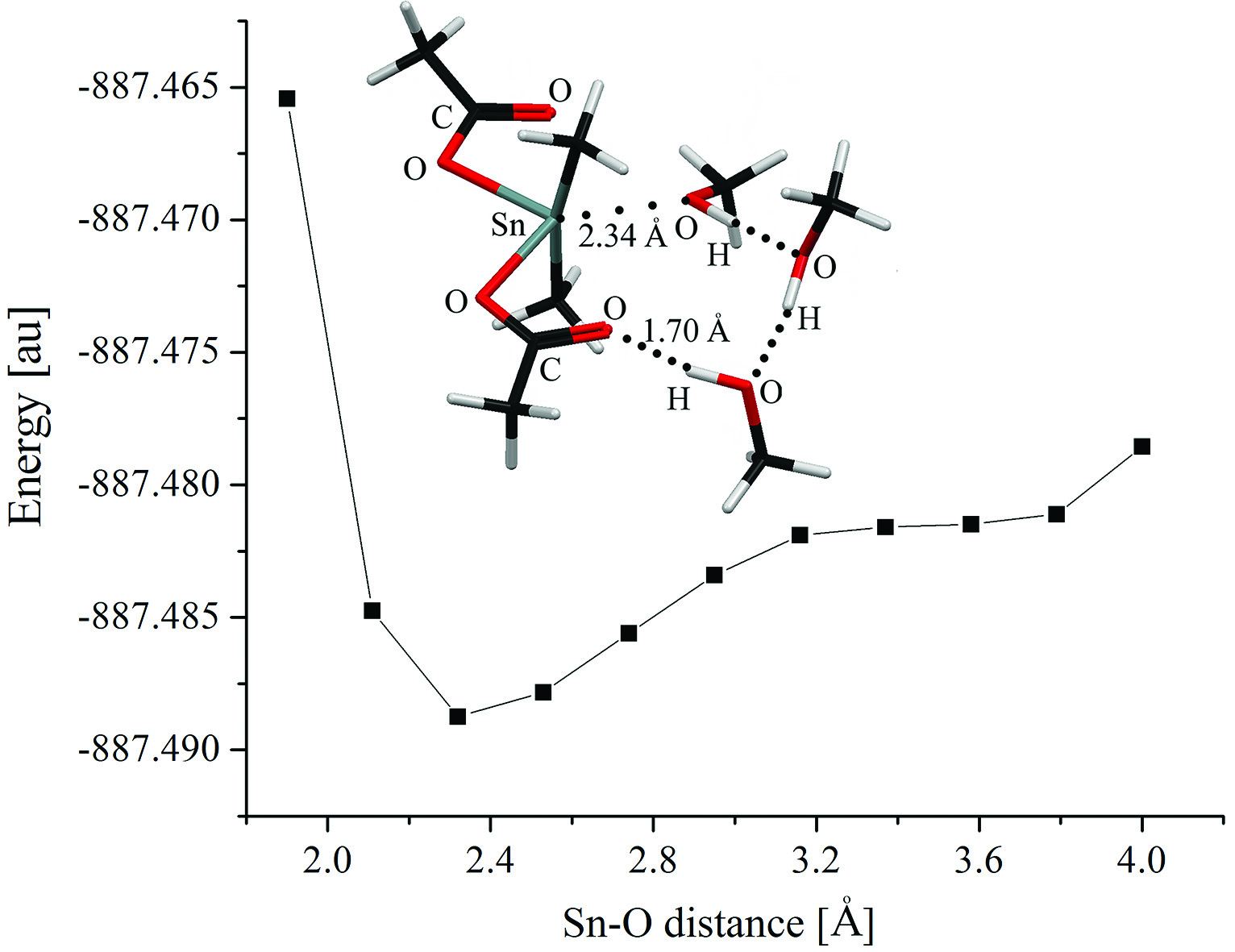
Figure 1: Computational modelling of the alcoholysis step of the reaction of Dimethyltin diacetate (DMTDA) with three methyl alcohol molecules.
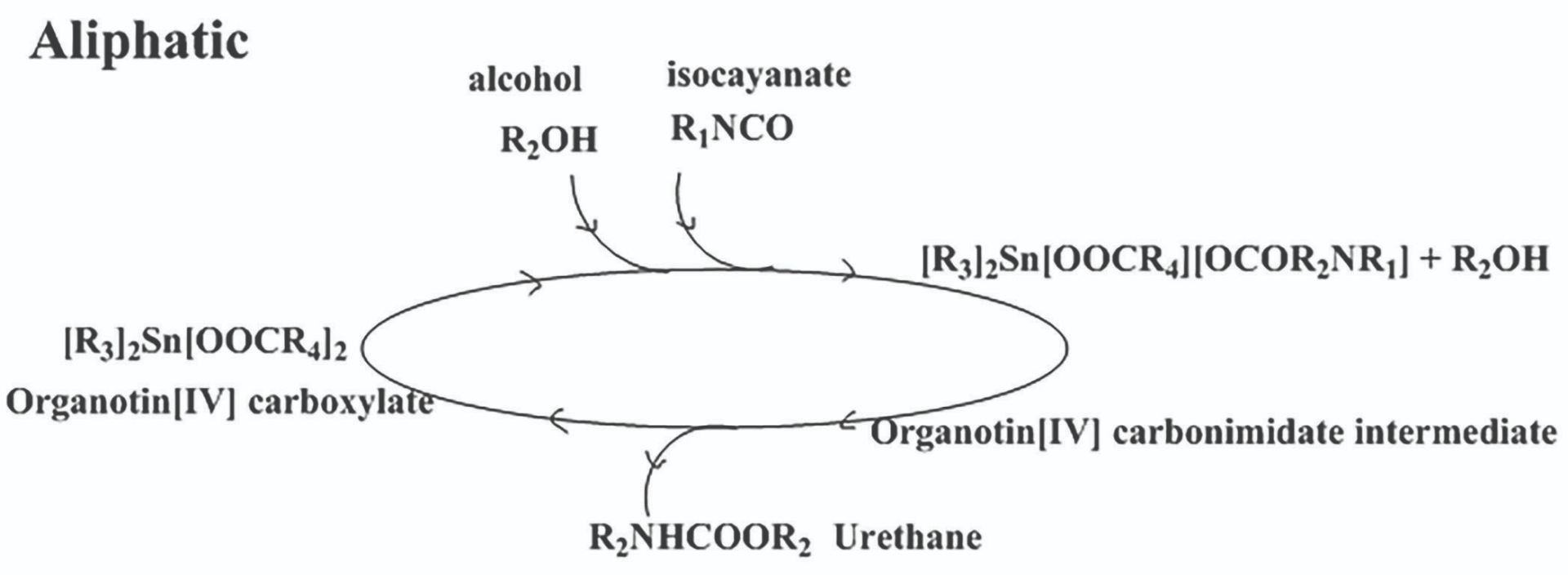
Scheme 1: Mechanism for urethane formation using aliphatic isocyanate with organotin carboxylate catalyst in a non-polar medium.
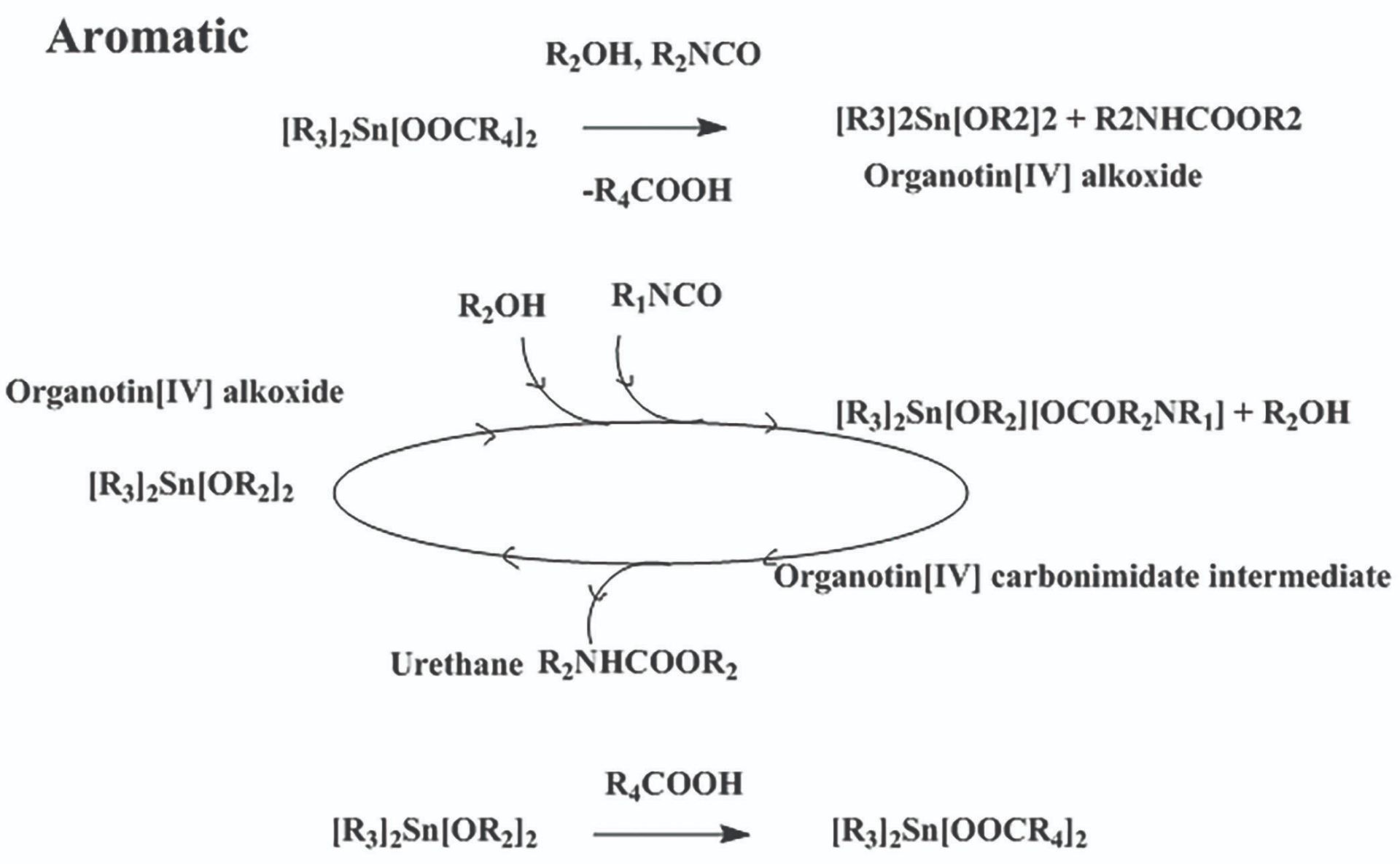
Scheme 2: Mechanism for urethane formation using aromatic isocyanate with organotin carboxylate catalyst in a non-polar medium.
These investigations were performed in a non-polar medium and showed that urethane formation for aliphatic isocyanates is different to that for aromatic isocyanates. For aliphatic isocyanates, the carboxylic ligand found in organotin carboxylate catalyst contributes to catalysis and for aromatic isocyanates it does not contribute to catalysis. The computational findings were supported by experimental work. Based on this data it was possible to propose mechanisms for urethane formation for aliphatic and aromatic systems in a non-polar medium. Schemes 1 and 2 show the proposed mechanisms for organotin carboxylate catalysed urethane formation for aliphatic and aromatic isocyanates. The findings were implemented in aliphatic, a pre-polymer manufacturing industry, to give coating systems with improved their performance. The products were marketed worldwide under a world leading surface coating brand.
Computational methods were also used to study interactions in different solvent environments and relate them to experimental results4 as well as understand specific interactions between organotin alkoxide and phenyl isocyanate that were reported in literature5. Investigations were also carried out for other metal catalysts such as titanium and zinc in urethane formation. The final aim of the investigation is to develop a tin free catalyst for aliphatic moisture cure polyurethane pre-polymer systems.
Student training
The project also has trained many undergraduates in the application of computational methods for understanding catalysis mechanisms for urethane formation. This will encourage students to select areas such as computational chemistry in their future studies.
References
- Szycher, M. Szycher’s Handbook of Polyurethanes, First Edition; Taylor & Francis, 1999.
- Gielen, M.; Davies, A. G.; Pannell, K.; Tiekink, E. Tin Chemistry: Fundamentals, Frontiers, and Applications; Wiley, 2008.
- Devendra, R.; Edmonds, N. R.; Söhnel, T. Journal of Molecular Catalysis A: Chemical 2013, 366, 126.
- Devendra, R.; Edmonds, N. R.; Söhnel, T. RSC Advances 2015, 5, 48935.
- Devendra, R.; Edmonds, N. R.; Söhnel, T. Journal of Molecular Catalysis A: Chemical 2014, 395, 72.
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































