
Quantifying gas narcosis in compressed gas diving
Dr Xavier CE Vrijdag; Dr Hanna van Waart; Professor Simon J Mitchell; Professor Jamie W Sleigh, Department of Anaesthesiology
Gas narcosis in divers
Divers venturing underwater either for work or pleasure, may experience narcotic effects caused by their breathing gases. The onset of narcosis symptoms is expected around 30 metres (405 kPa) when breathing air. The principal hazard of gas narcosis is euphoria, overconfidence, and loss of judgment. This may cause the diver to become less alert, take extra risks, and start a chain of events culminating in a severe diving accident. No early warning signal is available. Therefore, an objective method to detect narcosis could improve diver safety by characterising the related effects of each component in the breathing gas mixture, allowing the diver to make informed choices about gas mix composition, and facilitating related research.
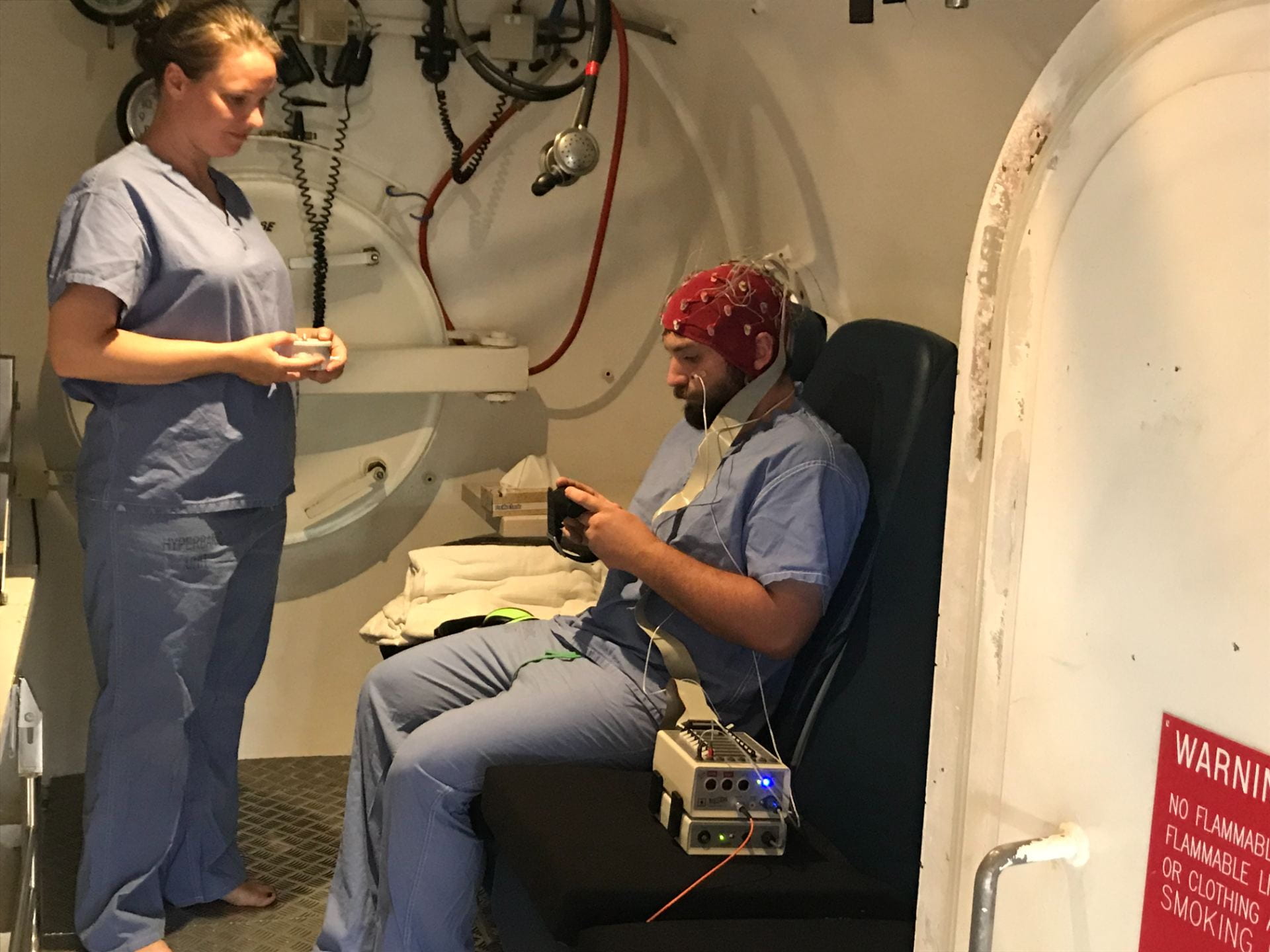
Figure 1: Participant seated inside the hyperbaric chamber, wearing the EEG cap connected to the headbox, with fibre cable to the laptop outside for data recording. He is performing the psychometric task on the dive computer. The inside attendant is holding the CFFF device. The breathing system is hanging above the participant, which is used in combination with a nose clip to administer the breathing gases.
Methods
In three studies involving twelve sport divers each, participants were exposed to: 1) three low-dose inspired fractions of nitrous oxide (20, 30 and 40%) blinded, in random order, as a normobaric research surrogate for hyperbaric nitrogen; 2) air and heliox (helium-oxygen mixture) at 284 and 608 kPa with the gases in random order, using heliox as a negative control gas expected to produce no narcosis; and 3) oxygen at 101, 142 and 284 kPa with the hyperbaric exposures in random order, to investigate any narcotic effects of hyperbaric oxygen. The cerebral effects were measured with pupillometry, critical flicker fusion frequency, psychometric tests, and EEG (Figure 1). Two novel EEG analysis algorithms were applied.
The complexity of the time course of the EEG was estimated using a novel metric designed in this program termed ‘temporal complexity’ which measures correlation between short successive EEG samples. With high correlation over time, the EEG signal is repeating itself, becoming simpler, indicating narcotic impairment (Figure 2).
The degree of spatial functional connectivity, estimated using mutual information, was summarised using the global efficiency network measure. Mutual information measures the information that is similar between two signals, calculated between all EEG channels. Higher mutual information means that the signals are more alike. Global efficiency measures how well connected a network of channels is. High global efficiency based on mutual information means that more signals are similar. Normal cognitive function requires regional specificity, which might be lost (that is, signals become more similar) during impairment.
Conclusion
These studies have provided two novel EEG analysis algorithms that quantify distinct cerebral effects of nitrous oxide, nitrogen, and oxygen, which might correlate with their different molecular mechanisms of action. Based on this research, divers can reduce their cognitive impairment by reducing their diving depth, increasing the oxygen content, or increasing the helium content in their gas mixture. This work is ongoing with additional data being collected to validate the algorithms and work towards a real-time version of the analysis algorithm. Ultimately, a qEEG method could be used as a continuous real-time monitoring system that is wearable underwater in a diving helmet. This system could warn the diver and supervisor of potential cognitive impairment, and as a result, reduce the chance of a serious diving accident.
Results
Pupillometry2 and critical flicker fusion frequency3 showed no differences between narcotic and non-narcotic conditions and were therefore not suitable to monitor gas narcosis.
In the nitrous oxide exposures, EEG temporal complexity correlated (r=0.50) with the psychometric test results in a mixed-effects model (p<0.001).4 Temporal complexity was decreased (signals were more repetitive) with higher nitrous oxide concentrations and lower cognitive performance. Exposure to hyperbaric air did not change the temporal complexity.5 This suggests that nitrous oxide and nitrogen cause their narcotic effect via different cellular pathways in the brain. It follows that although these gases may have similar behavioural effects, nitrous oxide is unsuitable as an experimental surrogate for hyperbaric nitrogen exposures.
Air-breathing at 284 (no noticeable narcotic effect) and 608 kPa (experienced as mild nitrogen narcosis) caused significant increases of 19% and 35% (respectively) in functional connectivity, compared to breathing air at the surface (p=0.050 and p=0.001) (Figure 3).6 Thus, signals exhibited greater similarity while breathing air at increased pressure. Heliox did not cause a significant increase in functional connectivity. This metric therefore exhibited specific sensitivity to nitrogen narcosis and is promising for further investigation of nitrogen narcosis in real-world diving.
Hyperbaric oxygen did not increase functional connectivity at 284 kPa suggesting oxygen cannot be narcotic in a similar way as nitrogen.7 This finding resolves a perennial debate among divers, some of whom believe oxygen may be more narcotic than nitrogen based solely on comparison of lipid solubility. Normobaric and hyperbaric oxygen at 101, 142 and 284 kPa caused a non-dose-dependent significant decrease in temporal complexity of 14, 16 and 16% compared to normobaric air breathing (p=0.010, p=0.003 and p=0.002 respectively). This might be an early indicator of oxygen hyperexcitability
Centre for eResearch support
The study data, including the EEG recordings, are stored on the University of Auckland Dropbox. This allows for easy collaboration between team members within and outside the University, easy secure access and sophisticated backups. Some EEG analysis are very computationally intensive. For these analysis, a virtual machine, hosted by the Centre for eResearch, has been used to expedite the analysis.
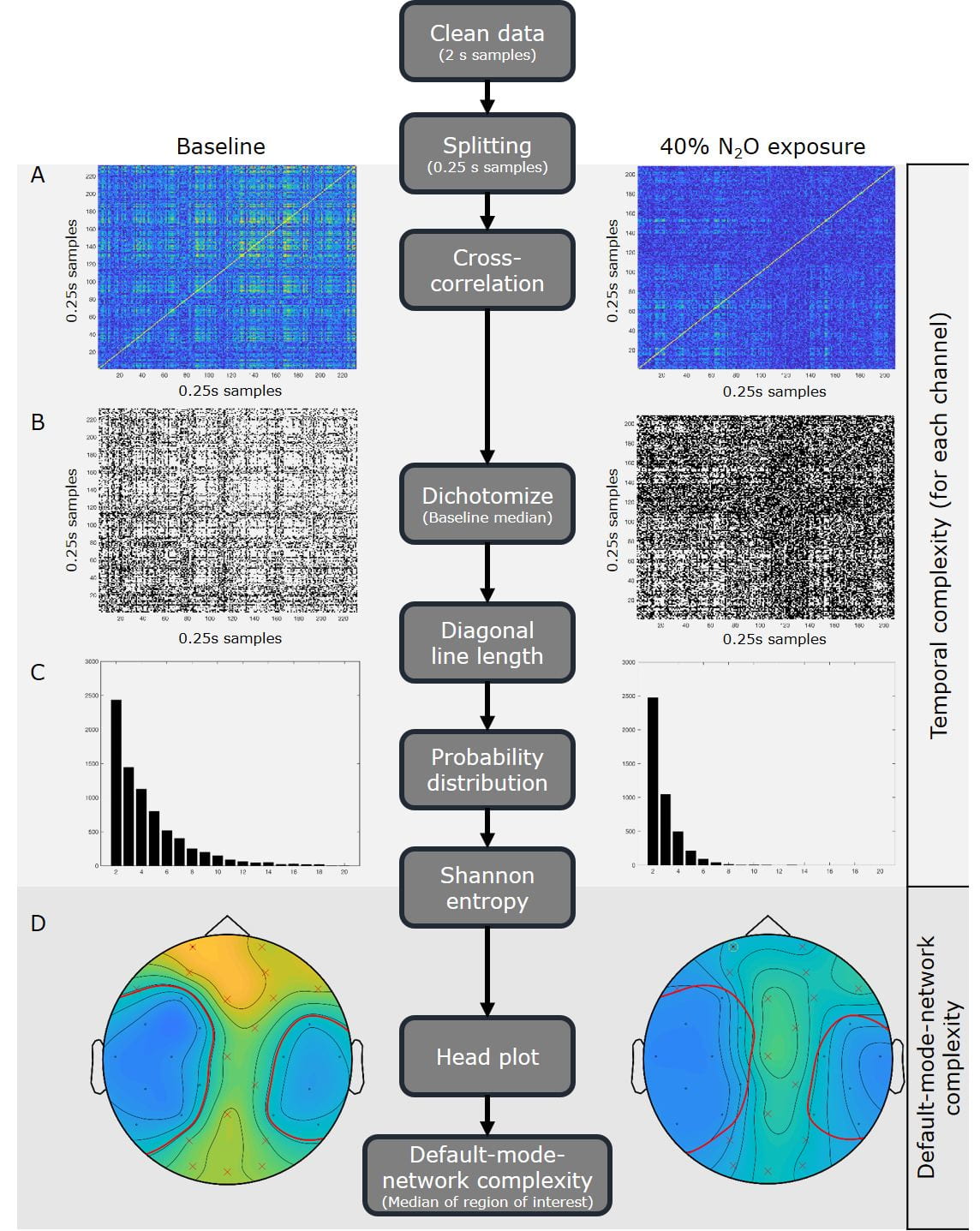
Figure 2: Flow diagram of the analysis algorithm in the middle. On the left, the intermediate results of the algorithm are shown of a participant during the baseline measurement. On the right, the results of the same participant during the 40% end-tidal nitrous oxide exposure. Panel A-C are from the signals of the Fp1 electrode (yellow circle in D). The analysis steps are repeated for each channel separately. Panel A shows the absolute cross-correlation values (blue low, yellow high) between the electroencephalogram (EEG) samples. Panel B shows the dichotomized results (0 black, 1 white). Panel C displays the probability distributions of the diagonal line lengths. Panel D is a surface head plot with contour lines of the Shannon entropy for all electrodes, showing the spatial distribution of temporal complexity (blue low, yellow high). Red line indicates the contour with value 1, in which the electrodes with a value above 1 at baseline (in yellow /green) are enclosed (red x). This selection is consistent over exposures and forms the region of interest for the EEG default-mode-network complexity analysis.
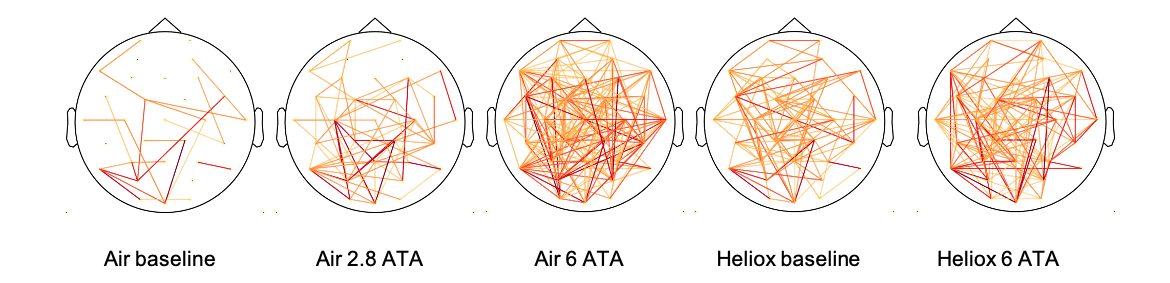
Figure 3: Head plots of binarized mutual information connections for each exposure. Yellow lines are common connections in more than three participants, while orange and red (>9 participants) depict connections seen in most of the participants.
This report is based on an earlier publication in the British Journal of Sports Medicine in their PhD award category.1
References:
1. Vrijdag XC. Towards gas narcosis monitoring in compressed gas diving (PhD Academy Award). Br J Sports Med. December 2022:bjsports-2022-106175. doi: 10.1136/bjsports-2022-106175.
2. Vrijdag XC, van Waart H, Sleigh JW, Mitchell SJ. Pupillometry is not sensitive to gas narcosis in divers breathing hyperbaric air or normobaric nitrous oxide. Diving Hyperb Med. 2020;50:115-20. doi: 10.28920/dhm50.2.115-120. PMID: 32557412.
3. Vrijdag XC, van Waart H, Sleigh JW, Balestra C, Mitchell SJ. Investigating critical flicker fusion frequency for monitoring gas narcosis in divers. Diving Hyperb Med. 2020;50:377-85. doi: 10.28920/dhm50.4.377-385. PMID: 33325019.
4. Vrijdag XCE, van Waart H, Mitchell SJ, Sleigh JW. An electroencephalogram metric of temporal complexity tracks psychometric impairment caused by low-dose nitrous oxide. Anesthesiology. 2021;134:202-18. doi: 10.1097/ALN.0000000000003628.
5. Vrijdag XCE. Monitoring gas narcosis in hyperbaric environments. University of Auckland; 2021.
6. Vrijdag XCE, van Waart H, Pullon RM, Sames C, Mitchell SJ, Sleigh JW. EEG functional connectivity is sensitive for nitrogen narcosis at 608 kPa. Sci Rep. 2022;12:4880. doi: 10.1038/s41598-022-08869-8. PMID: 35318392. PMCID: PMC8940999.
7. Vrijdag XCE, van Waart H, Sames C, Mitchell SJ, Sleigh JW. Does hyperbaric oxygen cause narcosis or hyperexcitability? A quantitative EEG analysis. Physiol Rep. 2022;10:e15386. doi: 10.14814/phy2.15386. PMID: 35859332. PMCID: PMC9300958.
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































