
Why are some molecules drugs?
Jóhannes Reynisson, School of Chemical Sciences
The fundamental question of why a few molecules are beneficial drugs whereas most molecules are just molecules is still unanswered.
By calculating the properties of known drugs using quantum chemical methods a region of properties can be established and used as a metric for drug design. For example, by analysing drugs with different oral activity (how well can the drug be administered as a pill) and
calculating their polarisabilities and dipole moments it is clear that a preferred region is favoured as shown in Figure 1.
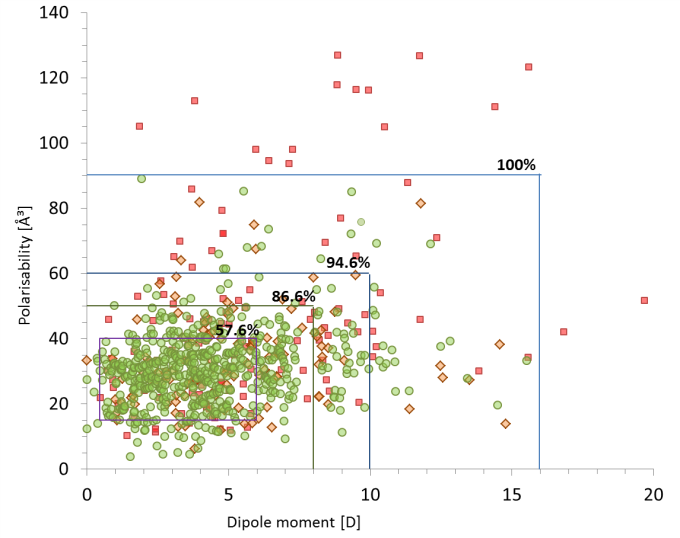
Figure 1: Dipole moment versus polarisability presenting the distribution of bioavailability groups with boundaries determined for Group. Group 1– low (red squares), group 2 – moderate (orange diamonds), group 3– high oral activity (green circles). In the range of 15-40 ų (polarisabilities) and 0.5 – 6 D (dipole moments) ~58% of high oral activity drugs are found meaning that this is a target area for designing new drugs with the ability to cross cell membranes.
Calculating the physiochemical properties of drug compounds
Having a computational resource such as the Pan cluster allows us to calculate the physicochemical properties of the drug compounds under study on a high level of theory. Gaussian software is used. A large host of drugs/ compounds need to be processed and the calculations required are computationally demanding. This is instrumental in defining the boundaries of Known Drug Space and without the Pan cluster this research is not possible. The next challenge in our research is to use the polarised continuum model (PCM) to predict the water solubility of drug compounds and establish its boundaries. The PCM method is a sophisticated theoretical approach that requires substantial computational resource to process all known drugs.
Papers published with data generated with NeSI resources
1. P.A. Hume, M.A. Brimble, J. Reynisson, Aust. J. Chem., 65 (2012) 402-408.
2. P.A. Hume, M.A. Brimble, J. Reynisson, Comp. Theor. Chem., 1005 (2013) 9-15.
3. K.L.M. Drew, J. Reynisson, Eur. J. Med. Chem., 56 (2012) 48-55.
4. B. Yu, J. Reynisson, Eur. J. Med. Chem., 46 (2011) 5833-5837.
See more case study projects

Our Voices: using innovative techniques to collect, analyse and amplify the lived experiences of young people in Aotearoa

Painting the brain: multiplexed tissue labelling of human brain tissue to facilitate discoveries in neuroanatomy

Detecting anomalous matches in professional sports: a novel approach using advanced anomaly detection techniques

Benefits of linking routine medical records to the GUiNZ longitudinal birth cohort: Childhood injury predictors

Using a virtual machine-based machine learning algorithm to obtain comprehensive behavioural information in an in vivo Alzheimer’s disease model

Mapping livability: the “15-minute city” concept for car-dependent districts in Auckland, New Zealand

Travelling Heads – Measuring Reproducibility and Repeatability of Magnetic Resonance Imaging in Dementia

Novel Subject-Specific Method of Visualising Group Differences from Multiple DTI Metrics without Averaging

Re-assess urban spaces under COVID-19 impact: sensing Auckland social ‘hotspots’ with mobile location data

Aotearoa New Zealand’s changing coastline – Resilience to Nature’s Challenges (National Science Challenge)

Proteins under a computational microscope: designing in-silico strategies to understand and develop molecular functionalities in Life Sciences and Engineering

Coastal image classification and nalysis based on convolutional neural betworks and pattern recognition

Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis

Measuring impact of entrepreneurship activities on students’ mindset, capabilities and entrepreneurial intentions

Using Zebra Finch data and deep learning classification to identify individual bird calls from audio recordings

Automated measurement of intracranial cerebrospinal fluid volume and outcome after endovascular thrombectomy for ischemic stroke

Using simple models to explore complex dynamics: A case study of macomona liliana (wedge-shell) and nutrient variations

Fully coupled thermo-hydro-mechanical modelling of permeability enhancement by the finite element method

Modelling dual reflux pressure swing adsorption (DR-PSA) units for gas separation in natural gas processing

Molecular phylogenetics uses genetic data to reconstruct the evolutionary history of individuals, populations or species

Wandering around the molecular landscape: embracing virtual reality as a research showcasing outreach and teaching tool
























































































































































